The rise and fall of e-cig startup Juul in the US, from Silicon Valley darling to a ban by the FDA
Travis Clark,Erin Brodwin
- The e-cigarette company Juul quickly became a Silicon Valley darling with a $38 billion valuation.
- But it went through several rebrands, investigations, and valuation slashes since.
The e-cigarette company Juul has hit a wall.
The company, valued at as much as $38 billion, staked a precarious path into the limelight, starting as a glitzy gadget and eventually billing itself as something resembling a public-health tool.
But its valuation had been slashed since then. Now, after several rebrands, controversies, and leadership shakeups, Juul products have been banned in the US.
On Thursday, the Food and Drug Administration ordered that Juul stop selling and distributing its products, and that its products currently in the market be removed.
Scroll down to see Juul's rise and looming fall:
2004: At Stanford, the product-design grad students James Monsees and Adam Bowen create the idea for Ploom, Juul’s precursor.

Monsees and Bowen have said they were smokers who met on smoke breaks while pursuing master's degrees in product design at Stanford University. Their thesis presentation, now posted on Juul's website, describes their product as "the rational future of smoking."
2007: Monsees and Bowen found the vaporizer startup Ploom in San Francisco.

By February 2008, Ploom raised $900,000 in venture funding, putting its valuation at roughly $3 million, according to PitchBook.
A 2011 description of the Ploom device, which sold for $75, described it as a heat-not-burn product that could be filled with single-serve refills called "Ploom Pods." The pods could include tobacco or non-tobacco ingredients, it said.
Aug. 1, 2013: Ploom debuts the Pax with a launch party in San Francisco.

After raising close to $5 million, Ploom launched a device called the Pax, a vaporizer for loose-leaf tobacco that could also be used for cannabis.
To debut the device, Ploom hosted a launch party in San Francisco's trendy Mission District.
At this time, Ploom investors included Japan Tobacco, the maker of Winston and Salem cigarettes, along with the software company Originate and the angel investment group Sand Hill.
Feb. 16, 2015: Monsees and Bowen sell the Ploom brand and a vaporizer line to the Japanese tobacco company JTI. They rebrand as Pax Labs.

As part of the deal, JTI said in a statement that Ploom would buy back JTI's minority stake in the startup.
June 1, 2015: Pax Labs launches the Juul with a party in New York City.
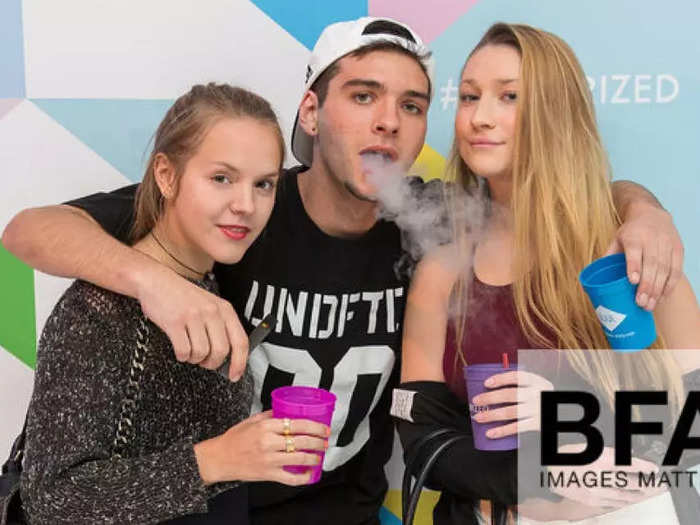
Pax introduced the Juul with a launch party in New York City.
A trove of images collected by Stanford researchers suggested that the campaign focused on a young audience. Guests were invited to try Juul's products free and share selfies on social media, Business Insider reported.
"Juul's launch campaign was patently youth-oriented," Robert Jackler, a practicing Stanford physician who was the principal investigator behind the tobacco-image collection, told Business Insider.
2016: Juul sales skyrocket 700%.

Juul devices gained popularity. Sales rose 700% in 2016, ABC 7 News reported.
July 1, 2017: Monsees and Bowen spin out Juul Labs as an independent company and name former Pax Labs CEO Tyler Goldman CEO.

Goldman came to Pax from the music-streaming startup Deezer then took over Juul Labs.
Nov. 2017: Juul is the best-selling e-cigarette on the market.

Juul said it'd sold 1 million units. The company also captured a third of the e-cigarette market, according to Nielsen data.
Dec. 11, 2017: CEO Tyler Goldman leaves Juul. The company replaces him with Kevin Burns.

Goldman left Juul to "pursue new entrepreneurial activities." The company hired Burns from the yogurt company Chobani.
Dec. 2017: Juul raises $112 million in venture funds and adds Nicholas Pritzker to its board, according to PitchBook.

The fresh funds came from firms including Tao Capital, Fidelity, and Evolution, according to PitchBook.
Nicholas Pritzker, Tao's cofounder, joined Juul's board, CNBC reported. Pritzker is a member of the wealthy Pritzker family, which owned the chewing-tobacco giant Conwood before selling it to the tobacco giant Reynolds. The Pritzkers also founded and expanded the Hyatt Hotels chain.
On an undisclosed date, Tao Capital sold its stake in Juul to the hedge fund Tiger Global and Manhattan Venture Partners, PitchBook said.
The venture fund M13, another early Juul investor, sold its shares in the spring of 2018.
This slide has been updated with new information about M13's investment.
March 2018: Dozens of outlets report that 'Juuling' is an epidemic at high schools.

National news outlets including National Public Radio, USA Today, and Business Insider reported that the Juul had a loyal and growing following among young people.
All of the reports said teens were taking to social media to brag about being able to sneak puffs in class or in the bathroom thanks to Juul's discreet design.
April 2018: Led by Commissioner Scott Gottlieb, the US Food and Drug Administration starts an 'undercover blitz' to crack down on sales of the Juul to minors.

In what the FDA said was the largest coordinated enforcement effort in agency history, the FDA issued more than 1,300 warning letters and fines to retailers who it said were illegally selling Juuls and other e-cigarettes to minors. The FDA found the retailers by conducting what it called "a nationwide, undercover blitz."
"Let me be clear to retailers," Gottlieb, then the FDA's commissioner, said in the statement, "this blitz, and resulting actions, should serve as notice that we will not tolerate the sale of any tobacco products to youth."
April 2018: Wall Street analysts warn that Juul is starting to encroach on Big Tobacco's financial terrain and could negatively affect Altria stock.

In a research note, Citigroup analysts warned investors that the Juul was beginning to disrupt tobacco stocks.
The note suggested that the rise of the Juul could bode poorly for tobacco companies — including Altria, British American Tobacco, and Imperial Brands — as sales were falling faster than expected.
"The US tobacco market is beginning to be disrupted by Juul," the analysts wrote, adding, "We don't expect underlying cigarette trends to improve much in the rest of 2018."
May 2018: Juul doubles its staff to 400 people.

June 2018: San Francisco bans flavored e-cigs like the Juul, prompting an endorsement from Michael Bloomberg.

Bloomberg, the former New York City mayor who is CEO of Bloomberg Philanthropies, called the move "an important step forward for public health" and said it should embolden other cities and states to follow suit.
July 8, 2018: Wall Street analysts say Juul is reviving the formerly comatose e-cig market, which had been slumping since 2014.

In a research note, Morgan Stanley analysts credited Juul with "driving a revival in the US e-cig market," adding that sales of Juul devices "accounted for almost the entire incremental increase in US e-cig sales as a percent of total cigarette and e-cigarette sales in the last year."
July 10, 2018: Juul raises $1.2 billion in a round that values the company at more than $16 billion, according to PitchBook.

The seven investors in the round included a maker of marijuana therapeutics, called Applied Biosciences, along with the the venture firm Bracket Capital, the hedge funds Darsana Capital and E Squared Capital, the investment giant Fidelity, the angel investor Sand Hill, and Tiger, according to PitchBook.
Aug. 21, 2018: Israel bans Juul products, calling them a 'grave risk to public health' because of their high nicotine content.

In a statement, Israel's Health Ministry said it's banning the sale and import of Juul devices because they contained more than 20 milligrams per milliliter of nicotine and presented "a grave risk to public health," Reuters reported.
Sept. 11, 2018: The FDA deepens its crackdown on Juul and other e-cig makers.

In a statement, then-Commissioner Gottlieb said the FDA was working on creating a system to "properly regulate" e-cigarettes like the Juul.
He said the aim was twofold: make e-cigarettes available as a less-dangerous alternative for adult smokers, but also keep them out of the hands of young people.
Oct. 2, 2018: The FDA surprises Juul at its headquarters and seizes 'thousands of pages of documents' as part of an investigation into its marketing practices.
The agency was running an investigation into whether Juul marketed its products to teens, CNBC said.
The visit was an extension of the FDA's request in April for materials related to how Juul presented its products and whether they were designed to appeal to kids, according to CNBC.
Oct. 2018: Juul surges in popularity, now accounting for over 70% of the US e-cigarette market, according to Nielsen data.

Nov. 13, 2018: Juul stops selling its sweet and fruity flavors at stores, making those varieties only available online.

Juul says it will temporarily stop selling its flavored e-cigarettes in stores.
The move comes on the heels of a similar ban on flavored e-cigs that the city of San Francisco enacted over the summer.
Researchers nearly unanimously praised the move, which they say could help protect young people by making the products less appealing and harder to purchase. Juul's flavored varieties will still be sold online, the company says.
Nov. 15, 2018: The FDA announces plans to curb flavored e-cig sales after reports that youth vaping has ballooned 78%.

2018: The Federal Trade Commission begins investigating whether Juul marketed its products to minors.

The Federal Trade Commission began looking into Juul's use of influencers and other marketing tools to appeal to young people, The Wall Street Journal reported in August 2019.
According to The Journal, the FTC's investigation began before it started reviewing a deal between Juul and the Marlboro maker, Altria, in December 2018.
Dec. 20, 2018: Altria buys 35% of Juul for $12.8 billion, bumping Juul's valuation to $38 billion. Gottlieb accuses both companies of backing away from pledges to curb youth vaping.

In what the Silicon Valley Business Journal called "the biggest investment ever in a US venture-backed company," Marlboro and the Parliament cigarette maker, Altria, paid $12.8 billion for a third of Juul. That gave Altria more combustible-cigarette market share than the next seven brands combined, according to the Centers for Disease Control and Prevention.
Juul, which had an annual revenue of about $2 billion at the time, also received a $2 billion bonus from Altria to distribute among its 1,500 employees, CNBC reported. That would have been about $1.3 million a person.
On the heels of the deal, Gottlieb called out both companies, saying they were backing away from previous pledges to fight teen vaping.
March 5, 2019: In a surprise announcement, Gottlieb announces he's leaving his post as FDA commissioner.

Gottlieb, a well-liked figure who spent just two years steering the country's top food and drug regulator, said he was leaving in a month to spend more time with his family in Connecticut.
The commissioner had made a name for himself as both a vocal critic of e-cigarette startups like Juul and a speedy approver of new pharmaceutical drugs.
In a resignation letter, Gottlieb wrote that one of his accomplishments at the FDA was taking actions against "bad actors that put Americans at risk."
March 13, 2019: Gottlieb announces a crackdown on flavored e-cig sales.

Roughly a week after announcing his departure from the FDA, Gottlieb released a plan to crack down on flavored e-cigarette sales at gas stations, pharmacies, and convenience stores. The plan would also crack down on websites without buffers against youth purchases, such as age-verification software or quantity limits.
April 3, 2019: The FDA says it's looking into a 'potential safety issue' related to seizures tied to vaping.

In a statement, Gottlieb said his agency had seen reports suggesting that a small number of e-cigarette users (35 cases from 2010 to early 2019) had experienced seizures after vaping.
By August, the FDA said it had received 127 reports — but noted that the new figure might simply mean more people were coming forward, not necessarily that cases were increasing.
Gottlieb also noted that seizures were known as possible side effects of nicotine poisoning and said the agency would continue exploring whether there was a connection.
April 8, 2019: Democrats in the US Senate launch an investigation into Juul's deal with Altria as well as its social media and advertising practices.

Eleven Democratic senators, including the party whip Dick Durbin and the presidential candidate Elizabeth Warren, wrote a letter to Juul demanding that the company answer questions about its advertising practices and its deal with Altria, CNBC reported.
June 13, 2019: The US House of Representatives announces an investigation of Juul's marketing and the Altria deal.

House Democrats launch their own investigation into Juul, Fortune reports.
July 16, 2019: Juul's CEO apologizes to parents of teens addicted to its vaping products.

In a CNBC documentary, Juul Labs CEO Kevin Burns issued an apology to parents of teens who were addicted to the company's vaping products.
"First of all, I'd tell them that I'm sorry that their child's using the product," Burns said.
July 25, 2019: Officials in Wisconsin warn of eight cases of severe lung disease in teens who'd vaped. It's unclear what kinds of products or substances are involved.

In July, Wisconsin's chief medical officer wrote a memo to healthcare providers warning them about a cluster of sick adolescents who had used e-cigarettes. Chest X-rays of the teens revealed similarities in lung damage, he says.
The following month, the CDC released an emergency notice about 30 cases of vaping-related lung illness in Wisconsin.
In mid-August, officials reported the first death tied to vaping-related lung illness: an adult in Illinois.
By September, the CDC and the FDA said there had been 530 confirmed and probable cases of the mystery illness since June. Seven people died. The investigation is ongoing, and officials have yet to find a substance or brand that's common among all the cases.
Aug. 16, 2019: Juul raises $785 million in equity and debt financing from Proioxis Ventures, according to PitchBook.

The funds will be used to speed Juul's expansion overseas, according to PitchBook. The figure brings the company to $14.2 billion in funds raised.
Aug. 29, 2019: Bloomberg says Juul devices were involved in three reports of seizures linked to vaping.

In three reports submitted to the FDA, people said they or their children had used a Juul before experiencing seizures, Bloomberg News reported. Bloomberg obtained the reports through a public records request.
In two of the three reports, the FDA wasn't able to officially confirm that a Juul device was involved, according to Bloomberg.
Aug. 29, 2019: Juul's CEO warns people against using Juuls and says vaping's long-term health effects are unknown.

Sept. 9, 2019: The FDA slams Juul for portraying its e-cigs as 'totally safe' and marketing them to kids at schools.
In a warning letter, the FDA said Juul wrongly painted its e-cigarettes, known in the industry as ENDS, as safer than cigarettes and marketed them intentionally to young people.
"Referring to your ENDS products as '99% safer' than cigarettes, 'much safer' than cigarettes, 'totally safe,' and 'a safer alternative than smoking cigarettes' is particularly concerning because these statements were made directly to children in school," the FDA letter said.
"Our concern is amplified by the epidemic rate of increase in youth use of ENDS products, including Juul's products," the letter added.
Sept. 17, 2019: Juul sales are halted in China for unclear reasons.

A selection of flavored Juul products that went up for sale on two online Chinese marketplaces, JD.com and Tmall, were removed within a week, The Wall Street Journal reported. Both retailers declined to say why.
Juul had long been planning to launch in China, where more than 300 million people smoke, according to the World Health Organization. Its nicotine refills, or Juul Pods, are manufactured in Shenzhen, China.
Sept. 18, 2019: India bans vaping, citing the "impact of e-cigarettes on the youth."

India outlawed the production, sale, import, and advertising of e-cigarettes, citing the need to stop the "impact of e-cigarettes on the youth," BuzzFeed News reported. Penalties include jail time and fines of up to $7,000.
Juul had been planning to launch in India, home to more than 106 million smokers — second only to China — by the end of 2019.
Sept. 23, 2019: The US Attorney's Office for the Northern District of California has reportedly launched a criminal investigation into Juul.

The Wall Street Journal reported that federal prosecutors in the US Attorney's Office for the Northern District of California were conducting a criminal investigation of Juul. Further details, such as the focus of the investigation, were not available, and Juul didn't respond to a request for comment from Business Insider.
Several other investigations are ongoing, including an investigation by the Federal Trade Commission focusing on whether Juul marketed to teens and an FDA investigation focused on marketing, outreach, and Juul's uniquely high nicotine content.
Sept. 24, 2019: Juul reportedly prepares to scale back its staff.

Juul began preparing to restructure its staff as it faced slower sales resulting from increasing reports about the mysterious vaping-related lung illness, the proposed US ban on flavored e-cigarettes, and a variety of other investigations, The Wall Street Journal reported.
The company employs roughly 3,900 people, according to The Journal, up from the 200 it had in 2017.
For now, Juul plans to hire less aggressively and start outlining plans to cut some jobs, according to The Journal, but will still continue to expand.
Sept. 25, 2019: CEO Kevin Burns steps down and is replaced by longtime tobacco executive K.C. Crosthwaite.

Crosthwaite was most recently chief growth officer at Altria, and has worked in tobacco for more than 20 years.
In announcing the change, Juul also said it would suspend US advertising and some lobbying efforts. Crosthwaite said he would "strive to work with regulators, policymakers and other stakeholders, and earn the trust of the societies in which we operate."
Oct. 7, 2019: A crop of school districts across three states sues Juul.

Four school districts sue Juul in what appears to be the beginning of a trend.
The districts include Three Village Central in New York, La Conner in Washington, Olathe in Kansas, and Francis Howell in Missouri. In separate suits filed on Monday, the districts argue that Juul created a public nuisance by intentionally marketing to kids; misrepresenting its products' nicotine content; and endangering teens' health, according to public documents that Business Insider viewed.
Cindy Ormsby, an attorney for the Missouri case, told the Riverfront Times that the Francis Howell lawsuit is "part of a coordinated package of litigation filed by school districts across the country, each dealing with a similar crisis of students addicted to nicotine."
In September, Kansas City school district Goddard became one of the first to announce that it was preparing a lawsuit against Juul.
The lawsuits seek unspecified damages and legal fees.
Oct. 17, 2019: Juul extends its ban on sweet and fruity flavors to include online sales.

Juul announces that it is stopping online sales of its mango, fruit, cucumber, and cream varieties. Last fall, the company temporarily banned sales of those varieties in stores. As of Oct. 17, those flavors can't be purchased in-person or online.
In a statement, Juul says it "will continue to develop scientific evidence to support the use of these flavored products."
Oct. 28, 2019: Juul reportedly plans to cut 500 jobs before year's end. Its chief marketing officer departs the following day.

Juul looks to eliminate roughly 500 jobs by the end of the year, the Wall Street Journal reports.
The cuts are part of a company-wide reorganization effort, according to the journal, and will involve anywhere between 10-15% of Juul's total workforce.
"As the vapor category undergoes a necessary reset, this reorganization will help Juul Labs focus on reducing underage use, investing in scientific research, and creating new technologies while earning a license to operate in the US and around the world," KC Crosthwaite, Juul's new CEO, said in an emailed statement provided to Business Insider.
The following day, the Journal reports that Juul's chief marketing officer is departing.
"Craig Brommers, an incredibly talented marketing executive, has asked to transition out of Juul Labs in the coming months so that he can pursue opportunities with other companies," a Juul spokesperson told the Journal.
The spokesperson also said that as a result of Brommer's departure, the CMO position would be cut.
Oct. 29, 2019: Juul names a new chief financial officer after its existing CFO asks to leave.

Juul appoints Guy Cartwright its new chief financial officer after CFO Tim Danaher asks to leave the company, the Wall Street Journal reports.
Cartwright previously served as managing director of the investment firm TowerBrook Capital Partners LP, and joined Juul in July, according to the Journal. Danaher had served as Juul's CFO since 2014.
Oct. 31, 2019: Marlboro maker Altria, which owns a third of Juul, slashes the value of its stake in the company. Juul is now valued at $24 billion instead of $38 billion.

Marlboro maker Altria, which last December purchased a 35% stake in Juul for $12.8 billion, cuts the value of its investment in the company by $4.5 billion.
The move reveals that Juul is now worth $24 billion, down from $38 billion.
On a conference call, Altria cited unexpected market shifts like regulatory crackdowns abroad and a proposed US flavor ban.
"We're not pleased to have to take an impairment charge on the Juul investment," Altria CEO Howard Willard said on the call. "We did not anticipate this dramatic a change in the e-vapor category," he added.
November 7, 2019: Juul stops selling mint flavored options, leaving only menthol and tobacco flavored refillable cartridges.

Juul announces it will stop selling mint-flavored refillable cartridges, or Juul pods.
In a press release, the company said it would immediately stop accepting orders for the mint-flavored pods from retail partners and stop selling mint-flavored pods online.
According to the release, Juul's decision was based partially on new research released this week which suggested that mint and mango were the most popular flavors among high school students who Juul.
"These results are unacceptable," Juul Labs CEO KC Crosthwaite said in the release, adding, "that is why we must reset the vapor category in the US and earn the trust of society by working cooperatively with regulators, Attorneys General, public health officials, and other stakeholders to combat underage use."
2020: Amid the pandemic, Juul lays off 40% of its workforce in April, 2020. It then lays off over half of its remaining staff, resulting in about a further 1,000 employees being cut.

2021: By 2021, Altria slashes its valuation for Juul to $5 billion, while Juul itself asserts it was worth $10 billion. Two years prior, the company was valued at $38 billion.

September 30, 2021: The CDC and FDA releases a study that finds that over 2 million middle-and-high-school students in the US were using e-cigarettes.

The study found that eight in 10 of those students used flavored e-cigarettes.
"These data highlight the fact that flavored e-cigarettes are still extremely popular with kids," said Mitch Seller, the director of the FDA's Center for Tobacco Products. "And we are equally disturbed by the quarter of high school students who use e-cigarettes and say they vape every single day."
February, 2022: A judge rules that Altria can keep its investment in Juul.
An administrative judge ruled that Altria didn't break antitrust laws by taking a 35% stake in Juul. The Federal Trade Commission had sued in 2019, and can still appeal the ruling.
June 23, 2022: The FDA bans Juul from selling and distributing its e-cigarette products in the US, and also ordered that all products currently in the market be removed.

The Wall Street Journal first reported on Wednesday that the FD could make the decision this week. Altria's stock plunged 10% on Wednesday after the Journal published its report.
READ MORE ARTICLES ON
Popular Right Now
Popular Keywords
Advertisement