- There are 176 potential COVID-19 vaccines in the works right now across the world.
- Of these, 35 are under clinical evaluation, according to the World Health Organisation’s vaccine update.
- Meanwhile, on September 14, the World Health Organisation reported a record number of COVID-19 cases.
It’s been ten months since the first coronavirus case was detected in China, and scientists around the world are still working to discover an effective vaccine. There are 176 potential COVID-19 vaccines in the works right now across the world. Of these, 35 are under clinical evaluation,
according to the World Health Organisation’s vaccine update. While top pharmaceutical companies like
AstraZeneca promises to launch vaccines by early 2021, it will still take at least four to five years until everyone on this planet is vaccinated.Meanwhile, on September 14, the World Health Organisation reported a record number of COVID-19 cases. So far, India has registered over 5 million COVID-19 cases, out of which 80,000 patients died.
The University of Oxford and AstraZeneca
Oxford
Clinical Stage: Phase III
AstraZeneca-Oxford’s vaccine candidate — ChAdOx1 — is the most advanced COVID-19 candidate in the world. Clinical trials for the most-advanced coronavirus vaccine candidate developed by AstraZeneca and the University of Oxford were put on hold on September 6, over safety concerns.
AstraZeneca has resumed trials in Brazil and the UK, but the late-stage trials will remain suspended in the US “for several days.” Despite the halt, the US-based pharma giant says its vaccine could be available this year or by early 2021. In India, Serum Institute too had to pause the trials, however, the Drugs Controller General of India today said it
can resume human trials again.
2. BioNTech/Fosun Pharma/Pfizer
Unsplash
CanSino Biological Inc. and Beijing Institute of Biotechnology
BCCL
Clinical Stage: Phase III
CanSino was the first pharma company in the world to get a go-ahead for the military use of an experimental coronavirus vaccine candidate. It was also the first company to start a clinical trial back in March. Can Sino Biologics is developing the potential coronavirus based on Adenovirus known as Ad5. The vaccine is under Phase III trials right now.
According to the Phase 1 and Phase 2 trial data released by The Lancet, CanSino’s COVID-19 vaccine is safe and can trigger an immune response in people. Chinese researchers claim the potential vaccine can protect people from “
all the known mutations of the virus.”
Gamaleya Research Institute
BCCL
Clinical Stage: Phase III
Russia’s potential COVID-19 vaccine, Sputnik V, is under Phase III trials. Gamaleya Research Institute — a part of Russia’s Health Ministry — is testing the virus on over 40,000 people.
Several scientists across the world have been criticizing Russia over safety concerns. According to Russian health minister Mikhail Murashko, one in seven volunteers had side effects after they had a shot of Sputnik V.
“Approximately 14% have small complaints of weakness, muscle pain for 24 hours and an occasional increase in body temperature,” reported Russian Agency TASS quoting Murashko.
On September 8, the Russian government sought India’s help in manufacturing the Sputnik V vaccine and also to conduct Phase III trials in India.
Janssen Pharmaceutical Companies
Unsplash
Clinical Stage: Phase III
Johnson and Johnson’s Janssen unit is planning started phase III clinical trials of its coronavirus vaccine. It will recruit upto 60,000 volunteers in US, South Africa, Brazil, Argentina, Columbia and Chile. It further said that we would know whether the one-shot vaccine works by the end of 2020,
Janssen Pharmaceuticals is among the many companies which have struck a deal with Britain. It has also struck a $1 billion deal with the US government for 100 million doses.
J&J aims to supply more than one billion doses of COVID-19 vaccine globally through 2021.
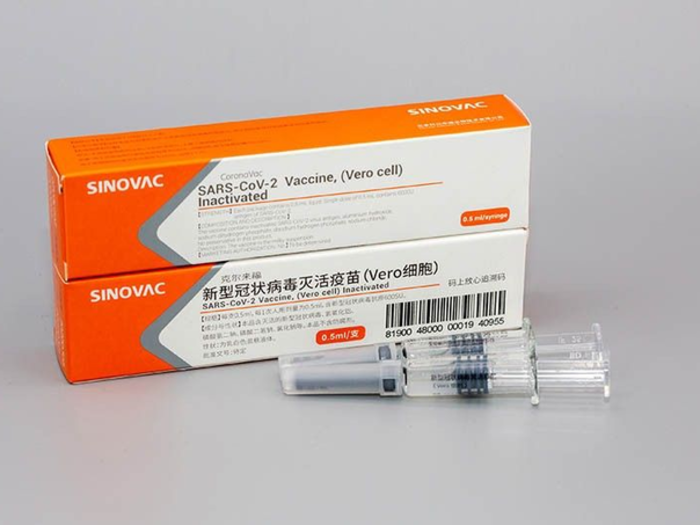
Sinovac
Chinese Embassy in Manila
Clinical Stage: Phase III
Chinese pharmaceutical Sinovac Biotech on July 16 got the approval to conduct Phase III clinical trial for its coronavirus vaccine candidate, CoronaVac. It started developing the COVID-19 vaccine as early as January 28. It aims to introduce its coronavirus vaccine this year.
Wuhan Institute of Biological Products/Sinopharm
BCCL
Clinical Stage: Phase III
Chinese pharmaceutical, Sinpharm, is the first in the world to start Phase III trials on a potential coronavirus vaccine. The vaccine is being developed in Wuhan — the same place where coronavirus first originated. Sinopharm is working on an “inactivated” vaccine. These vaccines are developed after scientists grow the virus in a laboratory setting from scratch — in this case, SARS-CoV-2 — and then try to kill it.
Beijing Institute of Biological Products/Sinopharm
BCCL
Clinical Stage: Phase III
Sinopharm is developing another coronavirus vaccine with the Beijing Institute of Biological Products. The inactivated vaccine is said to be available in the market by December for $150, reported Russian News Agency TASS. The vaccine is under Phase III trials right now.
The Beijing Institute of Biological Products claims that it can produce up to 120 mln inactivated vaccines a year.
Moderna/NIAID
BCCL
Clinical Stage: Phase III
Moderna is set to complete its Phase III trials by the end of September. It enrolled over 30,000 people in the US and started human trials. It aims to reveal the test results of the human trials by October and launch the vaccine by the end of the year. It is planning to manufacture 500 million doses of vaccine per year from 2021.