- Home
- slideshows
- miscellaneous
- What causes human bodies to age? Here's what scientists know about the biology behind growing old.
What causes human bodies to age? Here's what scientists know about the biology behind growing old.
Errors appear in DNA.

Gene expression goes awry.
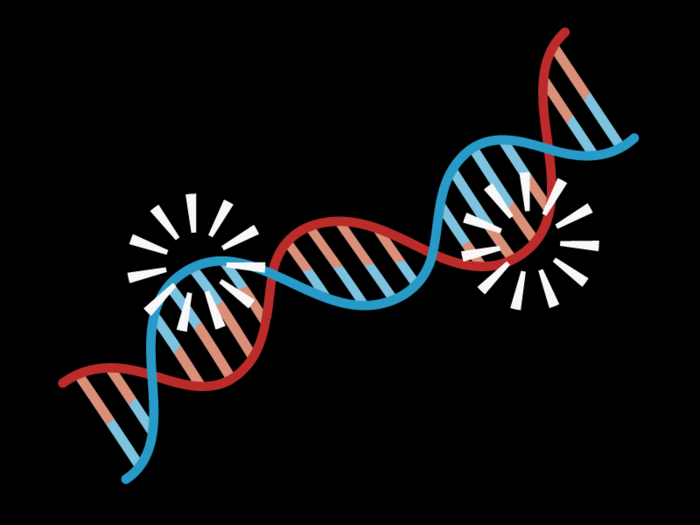
Certain parts of your DNA are read and translated into physical traits. A group of proteins in your cells controls which genes ultimately get expressed. This process is called epigenetic moderation, and it's what ensures your skin cells are different from brain cells, even though they use the same set of DNA.
But as we age, the proteins bound to DNA become looser and less accurate, and genes start to get expressed when they shouldn't be, or get silenced in error. This means some necessary proteins aren't being made, and harmful, unnecessary proteins are. For example, if an inadvertent change results in the silencing of a gene that helps suppress tumors, cells could uncontrollably grow into cancer.
Scientists have found that reversing these types of errors in gene expression can improve some neurological effects of aging in mice, such as memory impairment.
Telomeres may shorten.
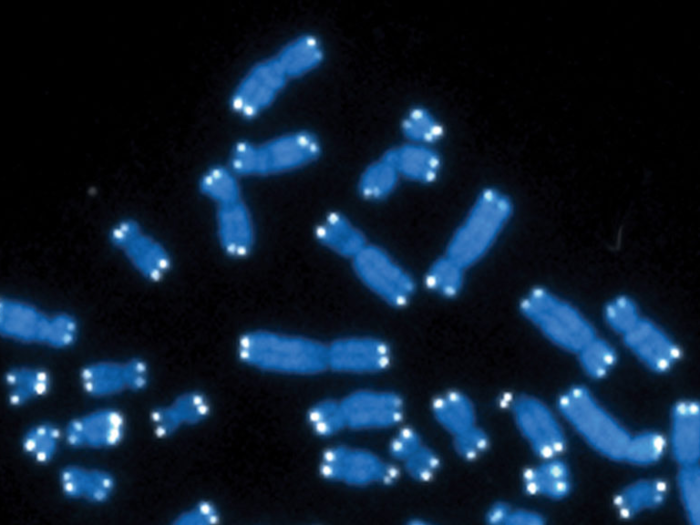
Telomeres are protective caps at the ends of each strand of DNA. Some scientists have compared them to the plastic tips of shoelaces that keep them from fraying.
Some research suggests that every time cells divide, the tips of the chromosome become shorter. When the telomeres are lost, chromosomes become unstable and all kinds of problems arise. The most notable is that chromosomes can't replicate correctly, and end up fragmented or with extra parts that aren't supposed to be there. These abnormalities usually kill cells or make them dangerous.
Scientists have figured out how to increase levels of telomerase – an enzyme that can extend the length of telomeres — in mice, and a study suggested that can extend mice's lifespan. When they lowered levels of telomerase in mice, the mice lived shorter lives.
Proteins become less stable and accurate in their roles.
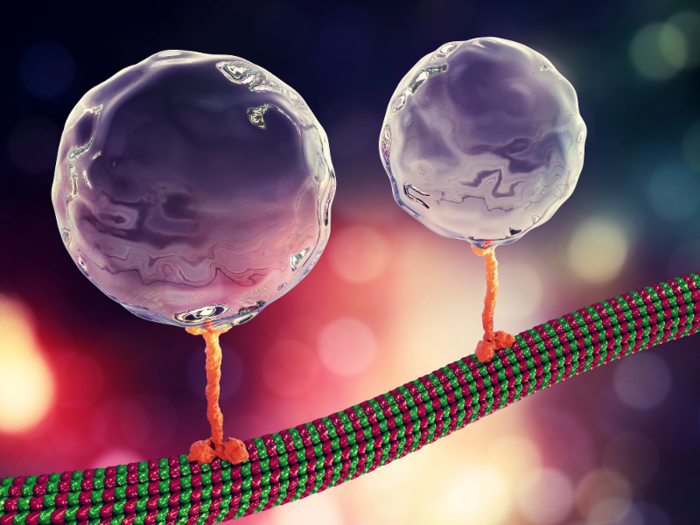
In our cells, proteins are produced constantly, and they control almost every function inside the cell. They move materials, carry signals, turn processes on and off, and provide structural support for the cell.
But proteins have to be recycled regularly because they lose their effectiveness over time. As we age, our bodies lose the ability to eliminate old proteins.
If our bodies can't turn over unusable proteins, they can build up and become toxic. Protein accumulation is one of the major features of Alzheimer's disease — proteins called beta-amyloid aggregate in the brain and result in the loss of nerve cells.
Cells don't die when they're supposed to.
As cells undergo stress and become damaged, they sometimes stop dividing and become resistant to death. They turn into something scientists call "zombie cells," which can infect other cells in their vicinity and spread inflammation throughout the body. These cells are also called senescent cells.
Senescent cells accumulate with time and age, and scientists have found that eliminating senescent cells in old mice seems to reverse some of the effects of aging. Similarly, when senescent cells were injected into young mice, they had debilitating and inflammatory effects, and were detrimental to overall health.
Several drugs called senolytics are now being developed with the goal of reducing senescent cells in the elderly to treat age-related disease.
The body's energy production machinery malfunctions.

Mitchondria produce energy in cells by converting oxygen and food into energy. As organisms and their cells age, these mini powerplants become more inefficient and dysfunctional. When they don't function properly, they can produce an altered form of oxygen that can cause damage to DNA and proteins.
In a study published in the journal Nature in June, scientists were able to reverse wrinkles in mice by restoring the function of their mitochondria.
Metabolism can become imbalanced.
Cells have to adapt to the amount of nutrients that are available. So if there is an imbalance with the cell's ability to sense or process nutrients, that causes problems.
With age, cells become less accurate at detecting the amount of glucose or fat that's in the body, so some fats and sugars don't get properly processed. Aging cells accumulate an excessive amount of fats not because older people ingest a lot of fat, but because cells don't digest it properly. This can affect the insulin and IGF-1 pathway, which play a role in diabetes.
This is why age-related diabetes is fairly common — older adults' bodies can no longer properly metabolize all the things they eat.
Tissues stop getting fixed and renewed.
Almost all tissues renew to some extent, but the rate of renewal becomes slower with aging, which is part of the reason why tissue damage accumulates.
Stem cells are cells that have the ability to become different types of cells in our body. In many tissues, they act as an internal repair system, replenishing cells that are damaged or dead. As humans age, stem cells become exhausted and less active, which means they can't divide as quickly. The exhaustion of stem cells means that tissues that are supposed to get renewed do not actually renew.
Cells become bad at communicating with one another.
For everything in the body to work, cells have to be constantly communicating with each other. They send signals through the blood and the immune system to do that. But as our bodies get older, cells become worse at communicating. Some cells become less responsive, which can turn them into inflammation-causing senescent cells. Inflammation produced by these senescent cells further blocks communication between healthy, functioning cells.
With cells unable to communicate, the immune system is unable to effectively clear out pathogens and senescent cells.
Aging also changes the level of inter-cellular communication across the endocrine and neuroendocrine systems. Messages sent through hormone molecules that circulate through these systems, such as insulin, tend to get lost.
In the end, the goal of research into the aging process is to find connections between these nine processes.

Scientists don't yet understand the connections between these nine agreed-upon hallmarks of aging. But according to Manuel Serrano, one of the authors of the paper, many scientists are now researching this.
"This is a proposal for further discussion," he told Business Insider. "Colleagues will start changing things, adding new topics and new connections."
When scientists understand enough about the science behind aging, they'll be able to create more effective treatments that can manipulate how we age and treat age-related diseases.
Richard Miller, the director of Glenn Center of Aging Research at University of Michigan, said that when it comes to manipulating aging, "the things that are going to really count are underlying control mechanisms that regulate multiple kinds of cellular events."
The real challenge, he believes, is figuring out what ties together all of the processes that cause our bodies to unravel.
Popular Right Now
Popular Keywords
Advertisement