- Home
- slideshows
- miscellaneous
- Wall Street thinks CBD could be a $16 billion industry by 2025. Here's what the cannabis compound does to your brain and body.
Wall Street thinks CBD could be a $16 billion industry by 2025. Here's what the cannabis compound does to your brain and body.
These days, CBD is everywhere. But its legality may depend on several factors, including the type of plant it comes from.

CBD has some limited — and very specific — clinical uses. But it's still unclear whether it could have health benefits for most people.
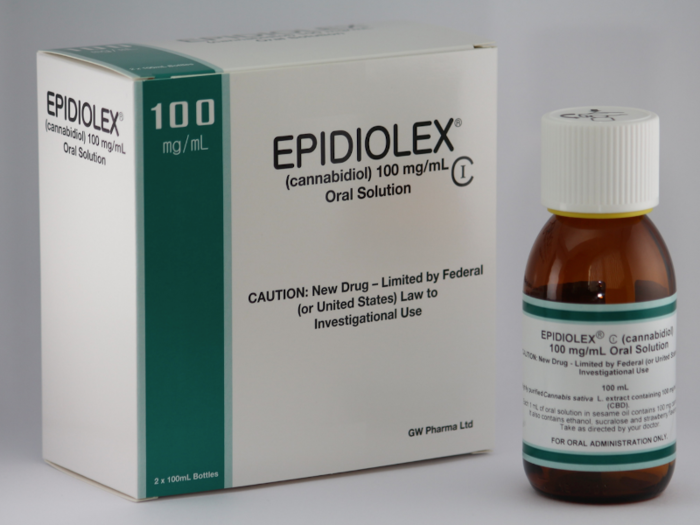
Last June, a prescription, CBD-based epilepsy drug called Epidiolex became the first of its kind to be approved by the federal government.
Taken as a syrup and made by British-based drugmaker GW Pharmaceuticals, the drug is designed to treat seizures linked with two rare forms of childhood epilepsy. The drug is made with CBD harvested from marijuana plants.
Epidiolex contains very high levels of CBD — far higher than the amounts found in treats and drinks being sold today. For example, the average dose for an 8-year-old child taking Epidiolex is roughly 650 milligrams of CBD per day. By comparison, most CBD cookies, coffees, and creams contain about 5-20 milligrams of CBD.
Still, many scientists believe CBD could play a role in helping to produce many of the therapeutic effects tied to marijuana.

In marijuana plants, CBD exists alongside THC, the ingredient that's believed to produce most of cannabis' commonly-known effects, including its characteristic high.
Researchers generally believe that when taken together in the plant, the two compounds produce their strongest effects. But they also believe that CBD alone could play a key role in everything from relieving pain to curbing inflammation.
These benefits have yet to be borne out by solid scientific research. Scientists are now exploring the potential of CBD to treat things like pain and inflammation.
New drug companies have been sprouting up with the goal of capitalizing on CBD's potential therapeutic effects.

Last fall, a pair of high-profile Stanford researchers launched a medical company to develop CBD-based therapies for conditions like arthritis, Crohn's disease, and multiple sclerosis.
Called Katexco Pharmaceuticals, the company is exploring whether CBD might help treat digestive conditions characterized by swelling and inflammation, such as Crohn's and irritable bowel disease. Katexco is based in Canada but has a US subsidiary in Silicon Valley.
Katexco joins several other startups that aim to help turn CBD into federally-approved pharmaceutical drugs. Part of their work could get easier when CBD can be made easily and cheaply in a lab, rather than from a plant.
Today, CBD comes from plants. But it can also be made in a lab — an advance that could cut costs and save time.

Instead of sourcing CBD from marijuana or hemp plants, researchers could make it in a lab.
In February, that happened for the first time. Using an increasingly popular approach known as synthetic biology, researchers at the University of California at Berkeley created the precursors to the cannabis compounds CBD and THC, and then made the compounds themselves — no farm or field required.
If the technique can scale, it could pave the way for making marijuana's therapeutic components more quickly and efficiently, for a fraction of the cost of traditional methods.
The Berkeley scientists licensed their lab-made cannabis technology to a startup they created called Demetrix. Several other startups are also pursuing the same goal, including Ginkgo Bioworks, a synthetic-biology startup in Boston; Intrexon, a biotech in Maryland; and Hyasynth Bio, a Canadian startup.
Wall Street believes the CBD industry could be worth $16 billion by 2025, depending on how federal regulators decide to police it.

In a February report, Wall Street analysts at the investment bank Cowen highlighted CBD as a massive opportunity for growth. The analysts estimated that the US market for CBD could skyrocket from roughly $1-$2 billion now, to $16 billion by 2025.
Survey results included in Cowen's report found that 7% of respondents said they were using a CBD supplement. The report also estimated that Americans spent up to $2 billion on CBD products last year. Ostensibly, folks are using CBD for everything from pain to anxiety. But again, those benefits have yet to be borne out by scientific research.
"This initial response piqued our interest considerably, as it was much higher than we would have expected," the analysts wrote.
Wall Street's CBD predictions could depend on how federal regulators decide to police the drug. For example, regulators could bar all CBD products, rule that cookies and creams can only contain a certain amount of of the compound, or allow the products to exist legally so long as they're derived from hemp.
The Food and Drug Administration is holding a hearing on Friday to discuss how cannabis products should be regulated to inform the agency's work in the growing market.
Regulators are gearing up to decide how to handle CBD, and a major hearing is happening on Friday.

The legal haze surrounding CBD could begin to clear as early as Friday, when federal regulators with the Food and Drug Administration hold a hearing on the compound.
Earlier this year during a Congressional hearing, former FDA Commissioner Scott Gottlieb suggested his agency would take a somewhat flexible approach to policing CBD products, cracking down when the products make health claims, when CBD-infused food products are sold across state lines, or when the products are marketed as dietary supplements.
But Gottlieb left his post in April, and a formal replacement has yet to be announced. Before his departure, however, Gottlieb created a working group to focus on cannabis regulation. That group is co-chaired by Principal Deputy FDA Commissioner Amy Abernethy and Principal Associate Commissioner for Policy Lowell Schiller. Ned Sharpless, the Acting FDA Commissioner, is leading Friday's hearing.
Popular Right Now
Popular Keywords
Advertisement