There is some good news for Indians — medicines showing promising results to treat coronavirus. India’s pharmaceutical companies like Glenmark, Hetero Drugs, and Cipla have repurposed at least five drugs that might help treat coronavirus patients.
These drugs were originally designed to treat other diseases like any skin disease or arthritis but now their generic version is introduced to treat coronavirus patients. The news comes at a time when the novel coronavirus has infected over 4 lakh people in the country and killed over 15,000.
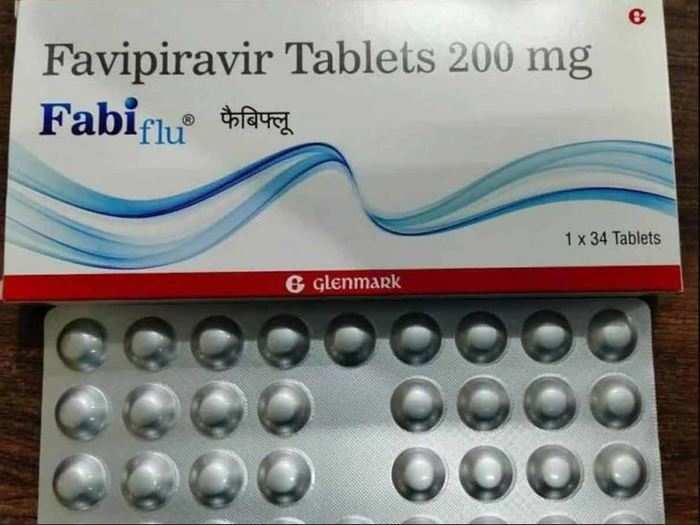
Fabiflu: Mumbai-based Glenmark is repurposing a drug called Favipiravir that was previously used to treat
BCCL
Glenmark Pharmaceuticals on June 22 announced the launch of antiviral drug Favipiravir — under its brand name FabiFlu — for the treatment of mild to moderate COVID-19 patients. Favipiravir — one of the most widely used drugs to treat coronavirus in the world — is an antiviral drug used to treat influenza.
Glenmark has received manufacturing and marketing approval from India's drug regulator, making FabiFlu the first oral Favipiravir-approved medication in India for the treatment of COVID-19.
The drug will be available at a price of ₹103 per tablet — for all those who have medical prescription — with a recommended 1800 mg dose twice on day one, followed by 800 mg dose twice from day 2 to day 14.
The drug will be available as a 200 mg tablet at a maximum retail price ₹3,500 for a strip of 34 tablets, Glenmark Pharmaceuticals said.
Covifor and Cipremi: Cipla and Hetero introduced the Remdesivir “the most promising candidate” for treating the novel coronavirus
BCCL
Pharmaceutical giants Cipla and Hetero introduced generic versions Remdesivir — an antiviral drug first used to treat Ebola — to fight coronavirus. India’s health Ministry approved use of the drug to treat mild cases of coronavirus. However, the drug is not recommended to treat those who have several renal impairment, pregnant and lactating
Woman, children below 12 years age.
Hetero said it will make its version of Remdesivir — Covifor — available between ₹5,000 to ₹6,000 for a vial. Whereas, Cipla is yet to decide the cost of Cipremi.
Cipla’s Cipremi is available for only those patients who are on oxygen support.
Apart from Cipla and Hetero Labs, Jubilant Lifesciences and Mylan are also working to manufacture the drug in India. Dr. Reddy’s Laboratories , Biocon firm Syngene and Zydus Cadila Healthcare have also received voluntary permission from Gilead Sciences to produce Remdesivir in India.
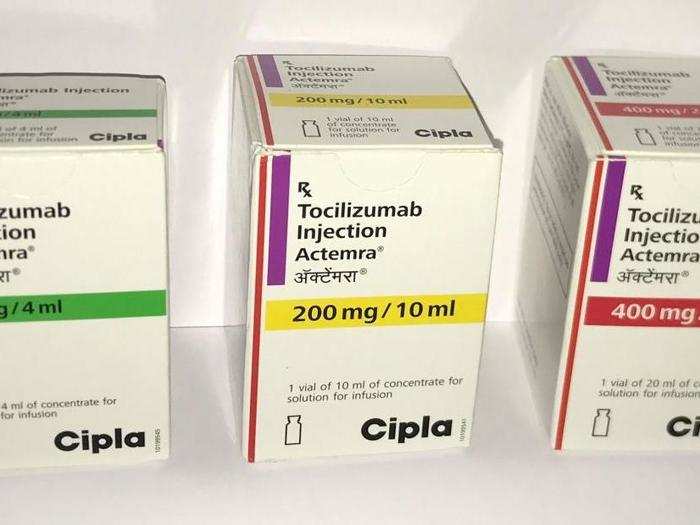
Tocilizumab and Itolizumab — drugs used to treat a disease where immune system mistakenly attacks your own body's tissue
Tocilizumab, a drug used to treat rheumatoid arthritis, is also being used in Mumbai to treat more than hundred severe cases of Covid-19. Tocilizumab is a drug produced by Roche pharma and marketed by Mumbai-based Cipla.
Another drug, Itlolizumab — a medicine used for those suffering with skin disorder psoriasis, rheumatoid arthritis, multiple sclerosis, and autoimmune disorders — is also used in Delhi and Mumbai. Itlolizumab is currently developed in India under a joint venture between Biocon and Centre for Molecular Immunology (CIM).
Sun Pharma is also working on a that was first developed to treat dengue. The company is conducting human trials on 210 patients across 12 centres and is expecting the results to be out by October this year.
Moreover, India is also experimenting with other treatments like plasma therapy by asking recovered covid-19 patients to donate their bloods to critical patients.
SEE ALSO:
Glenmark shares hit upper circuit twice as its COVID-19 drug enters phase-3 clinical trial
Better safe than sorry – the COVID-19 crisis could go either way and these are the shocks and surprises to prepare for